Good sleep changes everything
Just ask our millions of users.
At Resmed, we create life-changing health technologies that people love.


Dive into your sleep health
Learn more about the benefits of sleep, recommendations and our top tips.

Discover our lightest touch yet
AirTouch N30i™: Our luxurious-feeling new tube-up nasal mask with a fabric cushion and a frame that’s fully wrapped in fabric.
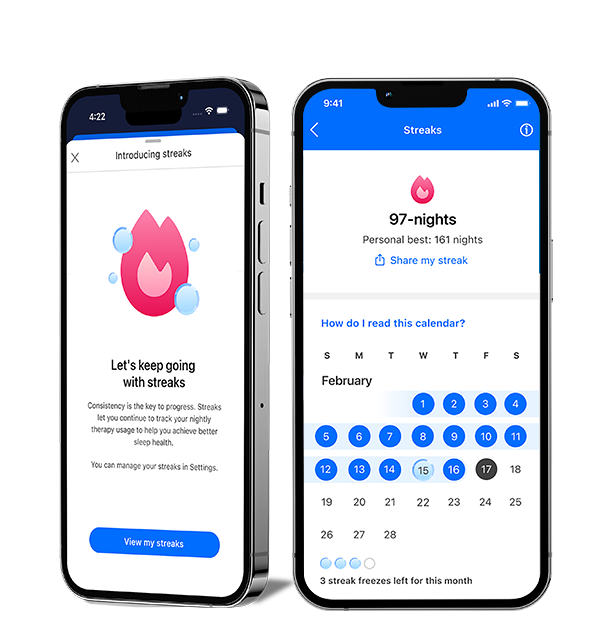
New to myAir: Streaks
See your progress and feel your momentum with the Streaks feature in myAir™. Easily track your consecutive nights on CPAP and celebrate your milestones. Start your streak tonight and set new records.

Residential Care Software
Our Residential Care Software team offers innovative, comprehensive software platforms that support healthcare providers in settings outside of the hospital.
Stay in the know
Be among the first to hear all the latest news and developments.









