Research in sleep-disordered breathing
An expanding body of scientific evidence clearly indicates that sleep-disordered breathing (SDB) has a profound negative impact on public health. Through its strong partnership with the global medical community, ResMed is committed to:
- Increasing awareness of the dangers of untreated SDB
- Supporting ongoing research into the correlation between SDB and other health risks
- Finding effective clinical solutions to improve the health and lives of SDB patients
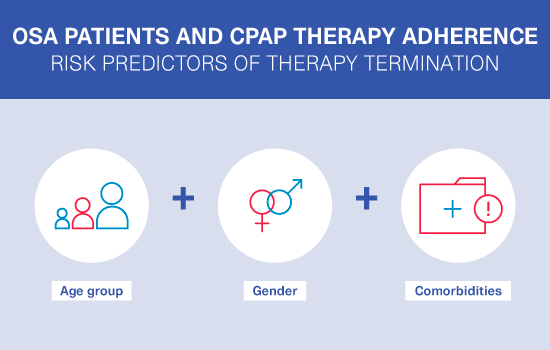
ALASKA - “A nationwide cLAimS data laKe for sleep Apnoea"
A large-scale analysis of the French national healthcare database (SNDS*), which explored the patient profiles and PAP adherence of almost half-a-million sleep apnoea patients during their first three years of PAP therapy.
ALASKA study outcomes:
first publication1
ALASKA showed that it’s important to consider patient phenotyping and personalised care when developing integrated sleep apnoea management strategies.
Our infographic highlights the key findings.
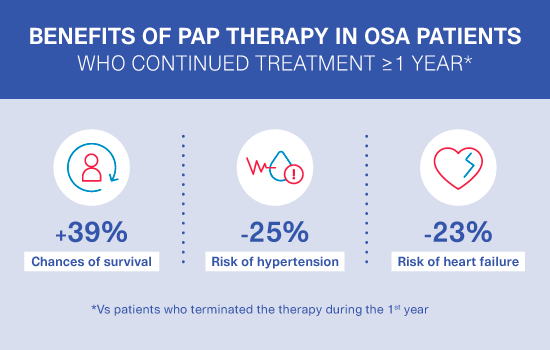
ALASKA study results:
second publication2
Chances of survival increased by 39% and the risk of developing heart failure and hypertension reduced for patients continuing PAP treatment throughout the first year: ALASKA’s second publication followed 176,014 PAP patients in the French healthcare system database over 3 years.
For more, download our infographic.
Central sleep apnoea research
Support for investigator initiated research
ResMed believes in the need to support ethical, independent clinical research, conducted by qualified third-party investigators.
*Système National des Données de Santé | SNDS – www.snds.gouv.fr
**Please note the Narval CC mandibular advancement device is not available in England, Scotland or Wales.
Narval CC is indicated to treat adults with snoring or mild to moderate obstructive sleep apnoea (OSA). In cases of severe OSA, it is indicated after continuous positive airway pressure (CPAP) therapy failure, non-compliance or refusal.
This content is intended for health professionals only. Please refer to the instructions for use for relevant information related to any warnings and precautions to be considered before and during use of the product.
- Pépin, J.-L.; Bailly, S.; Rinder, P.; Adler, D.; Szeftel, D.; Malhotra, A.; Cistulli, P.A.; Benjafield, A.; Lavergne, F.; Josseran, A.; et al. CPAP Therapy Termination Rates by OSA Phenotype: A French Nationwide Database Analysis. J. Clin. Med. 2021, 10, 936. https://doi.org/10.3390/jcm10050936 Retrospective analysis on 480 000 adult patients with CPAP therapy initiated from 2015 to end of 2016, identified in the French Health insurance claims database, and followed up until end of 2019
- Pépin JL, et al. Relationship between CPAP termination and all-cause mortality: a French nationwide database analysis, CHEST (2022), doi: https://doi.org/10.1016/j.chest.2022.02.013Retrospective analysis of 176 014 adult patients identified in the French health insurance claims database, who started PAP therapy between January 2015 and December 2016 and were followed up for 3 years