Central sleep apnoea during CPAP therapy: first insights from a big data analysis
Big data is a promising and innovative way to explore questions of clinical relevance, identify disease patterns and characteristics, and generate hypotheses in healthcare. A tremendous amount of data is now available -and growing exponentially- from a number of sources, including telemonitored medical devices that are connected to databases and provide information on device performance and patient status. Analysis of such data may provide new insights and support new approaches to healthcare management.
In a cutting-edge analysis, real-world, de-identified data were used to characterise central sleep apnoea (CSA) during continuous positive airway pressure (CPAP) therapy of US telemonitored patients. The analysis was able to identify 3 categories of CSA during CPAP therapy, all of which negatively affected CPAP therapy compliance and increased therapy termination risk.1
A second analysis, performed on the same database, found that switching patients with persistent or emergent CSA from CPAP to ASV therapy* may improve compliance and could thus potentially improve patient outcomes.2

Three categories of CSA during CPAP therapy were identified: emergent, transient, and persistent CSA
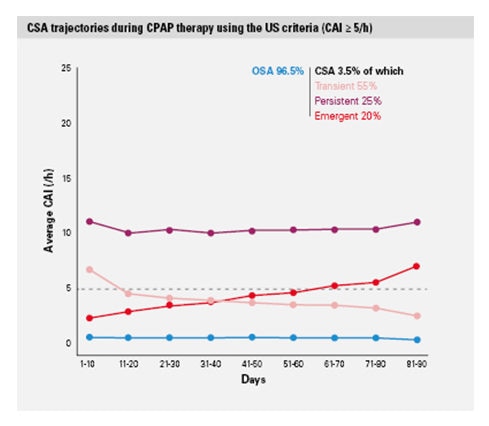
The study “Trajectories of CSA during CPAP therapy” looked at de-identified data from 133,000 telemonitored patients treated for sleep disordered breathing (SDB) with ResMed positive airway pressure (PAP) devices in the US in 2015.1 New information about the natural history of CSA during CPAP therapy was discovered using repeated measures based on real-life telemonitoring data rather than single “snapshots” of CSA.
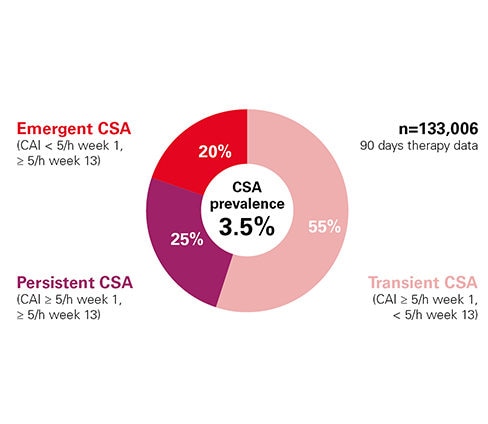
CSA occurred in 3.5% of patients; 3 categories of treatment associated CSA were identified1: emergent (20%), transient (55%), and persistent (25%) CSA.
Each category is associated with decreased compliance and increased therapy drop-out risk1
- The presence of CSA was associated with decreased CPAP usage hours and increased likelihood of treatment discontinuation, as compared with OSA. The probability of continuing CPAP therapy on day 300 was 83% for OSA, and 79%, 76% and 72% for transient, persistent and emergent CSA, respectively.
- The hazard ratios for therapy termination for the 3 CSA groups were 1.3, 1.5, and 1.7, respectively.
- These findings were consistent using either the European Respiratory Society or the US definition of persistent CSA (AHI ≥15/h or Central Apnoea Index (CAI) ≥5/h).
Switching from CPAP to ASV in patients with emergent or persistent CSA may improve adherence2
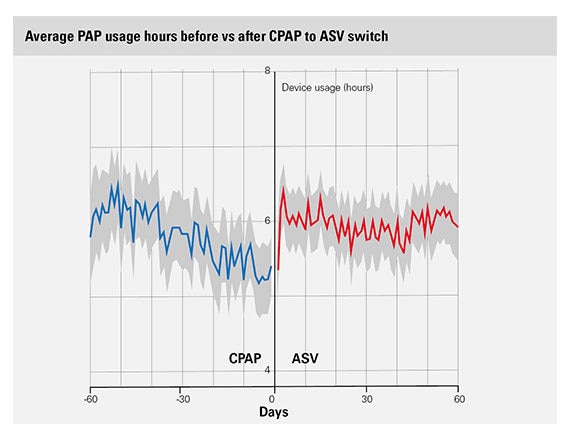
A secondary analysis showed that compliance in patients with emergent or persistent CSA who switched from CPAP to ASV improved immediately after the switch was made. There was a +22% adherence improvement in the two patient subgroups that switched from CPAP to either fixed (n=127, p <0.05) or variable (n=82, p <0.01) EPAP ASV.2
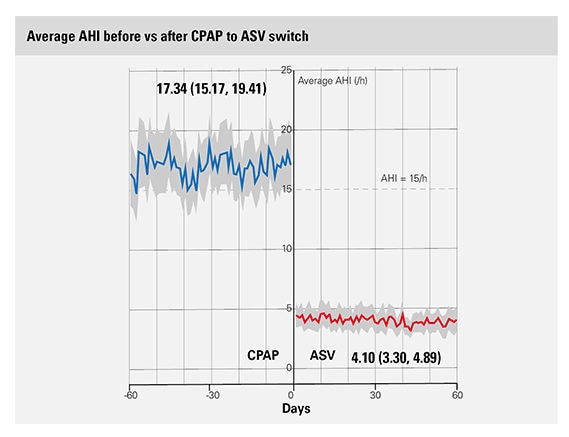
The average AHI before the CPAP to ASV switch among patients with emergent or persistent CSA was 17.34/hour as compared with 4.1/hour after the switch.
The data suggest that if CSA persists after 2 weeks, the patient fits within the trajectory of emergent or persistent CSA and may benefit from a switch to ASV.*
The study was led by an external international committee of sleep and respiratory experts: Jean-Louis Pépin (France), Holger Woehrle (Germany), Atul Malhotra (USA), and Peter Cistulli (Australia).
Big data studies – experts videos
Watch experts talking about big data study
Big data analysis: main findings
Holger Woerhle, MD, explains the main findings of a big data analysis on CSA during CPAP therapy.
Trajectories of Emergent CSA during CPAP therapy
Professor Jean-Louis Pépin explains the findings of the big data analysis “Trajectories of Emergent Central Sleep Apnoea during CPAP therapy”.
Big data: the next frontier in respiratory and sleep medicine?
Ramon Farré, PhD, shares perspectives regarding big data: definition, new analytical concepts and tools, patient privacy protection, regulatory perspective and risks.
Implications for clinical practice
Identify residual CSA with ResScan
The statistical data of ResScan provide the AHI, AI, CAI, Hypopnea Index (HI) and Oxygen Desaturation Index (ODI) (if used with an oximeter), which allows you to identify residual CSA and Cheyne-Stokes respiration (CSR) during CPAP therapy.
- Log into ResScan
- Go to patient record
- Go to “Settings”
- Check that your patient is on CPAP/APAP therapy by looking at the Therapy mode
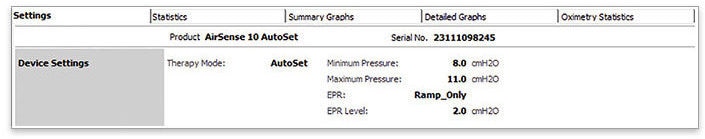
- Go to “Statistics”
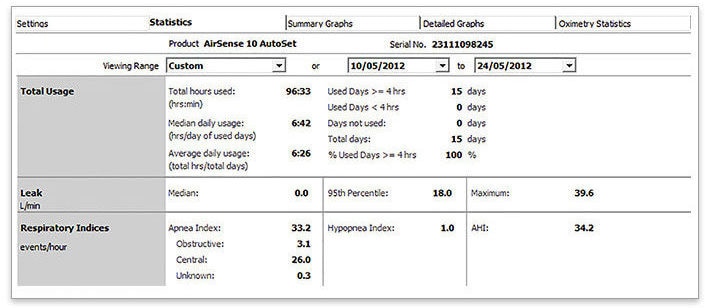
- Select the last 2 weeks
- Look at AHI & AI to see if: AHI ≥ 15/h, AI > 5/h or CAI > 5/h
Identify residual CSA with AirView
AirView‘s colour-coded wireless dashboard allows you to easily identify low usage and residual AHI. It provides an overview of the usage, AHI and leakage of the last 10 days. AirView icon guide is available here.
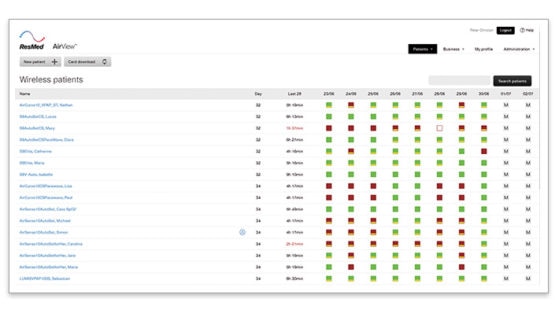
1. Log into AirView
2. Go to the patients tab and then click on wireless selection to access the wireless dashboard
3. Look at your patients on CPAP/APAP therapy
4. Look at patients with these icons
5. Click on the square indicating a too high AHI to see detailed data
- Look at AHI to see if AHI ≥ 15/h
- Click on the patient
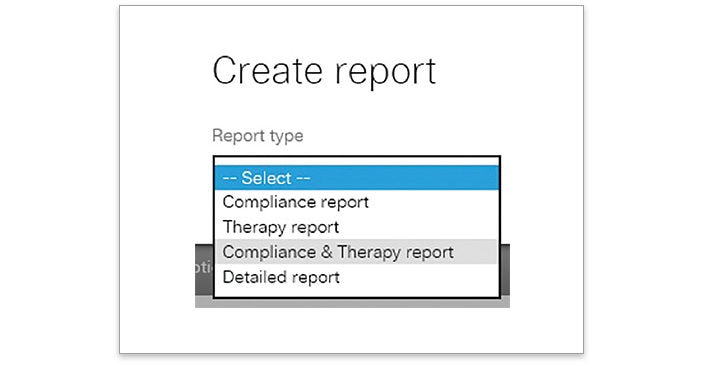
- Go to “Create report” and select “Compliance & Therapy report”
- Go through the report and look for CAI to see if CAI > 5/h
Support for investigator initiated research
ResMed believes in the need to support ethical, independent clinical research, conducted by qualified third-party investigators.
More about central sleep apnoea

SERVE-HF study
SERVE-HF has become a key study in its field, contributing significantly to ASV clinical practice.

FACE study
The FACE study will provide long-term data on morbidity and mortality of heart failure (both HFrEF and HFpEF) patients using ASV.

Treatment options for CSA
It can be challenging to treat patients with central sleep apnoea. Learn more about CSA treatment options.
References:
*ASV therapy is contraindicated in patients with chronic, symptomatic heart failure (NYHA 2-4) with reduced left ventricular ejection fraction (LVEF ≤ 45%) and moderate to severe predominant central sleep apnoea.
- Liu et al. Trajectories of Emergent Central Sleep Apnea During CPAP therapy. Chest. 2017;152(4):751-60.
- Pépin et al. Adherence to Positive Airway Therapy After Switching From CPAP to ASV: A Big Data Analysis. J Clin Sleep Med. 2018 Jan 15;14(1):57-63. doi: 10.5664/jcsm.6880.