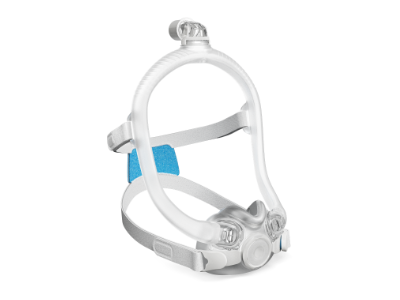
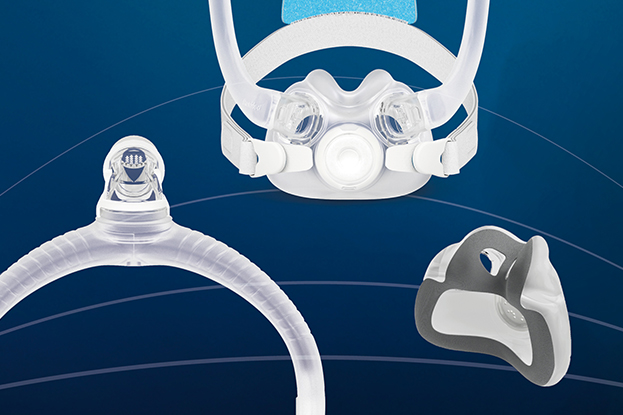
AirTouch F30i Clear
- A full face mask designed to deliver what patients need to stay on therapy: a soft and comfortable seal, reliable fit and freedom to move.
- Patients say the AirTouch™ F30i Clear is very comfortable overall1.
- Available in three cushion sizes and featuring AdaptiSeal™ technology, each cushion is designed to contour to different facial structures and support a secure, reliable seal.
- The ComfiSoft™ cushion is interchangeable with AirFit™ F30i and AirFit X30i cushions.
- Available for sale only in the US

Softness where patients feel it most
The ComfiSoft™ cushion with AdaptiSeal™ technology delivers softness where patients feel it most — at the seal. For providers, that means patients with better first-time fits and fewer adjustments.
Crafted for everyday therapy
The top-of-the-head tube connection with 360° elbow rotation is designed to help patients move freely in their sleep — on the side, back or stomach — without the tubing pulling or getting in the way. Magnetic clips* and a quick-release elbow make it simple to put on, take off or pause therapy without hassle.

Designed for quieter nights to help patients stay on therapy
At just 20 decibels2 — softer than a whisper — this mask’s QuietAir™ venting is designed to keep sleep peaceful for patients and their partners. A quieter bedroom may help create a more comfortable experience for patients and their partners, supporting ongoing therapy use.
* The mask contains magnets that may interfere with certain implants or medical devices. Please refer to the User Guide for complete labeling information, including magnet contraindications and warnings.
References
- Source: Resmed conducted an external crossover (or single-arm) study of the AirTouch F30i Clear mask, using the AirFit F30i as a benchmark. The study involved 33 adults who had been on PAP therapy for OSA (≥ 6 months) and were using other full-face masks, including the AirFit F30i (n=22), AirFit F20 (n=3), AirTouch F20 (n=3), AirFit F30 (n=2), AirFit F40 (n=2), DreamWear Full Face (n=1), and Mirage Quattro (n=1). The study took place in Sydney, Australia, from March 17 to April 7, 2025. Note for claims vs Own masks based on ECS 3: The study started with n=34, with n=33 completed the study AND only n=33 was used for Mask A vs Own Mask p value calculation (only paired data).
- Source: Resmed internal testing for the sound power of AirTouch F30i mask with QuietAir vent, ID: A5637990.